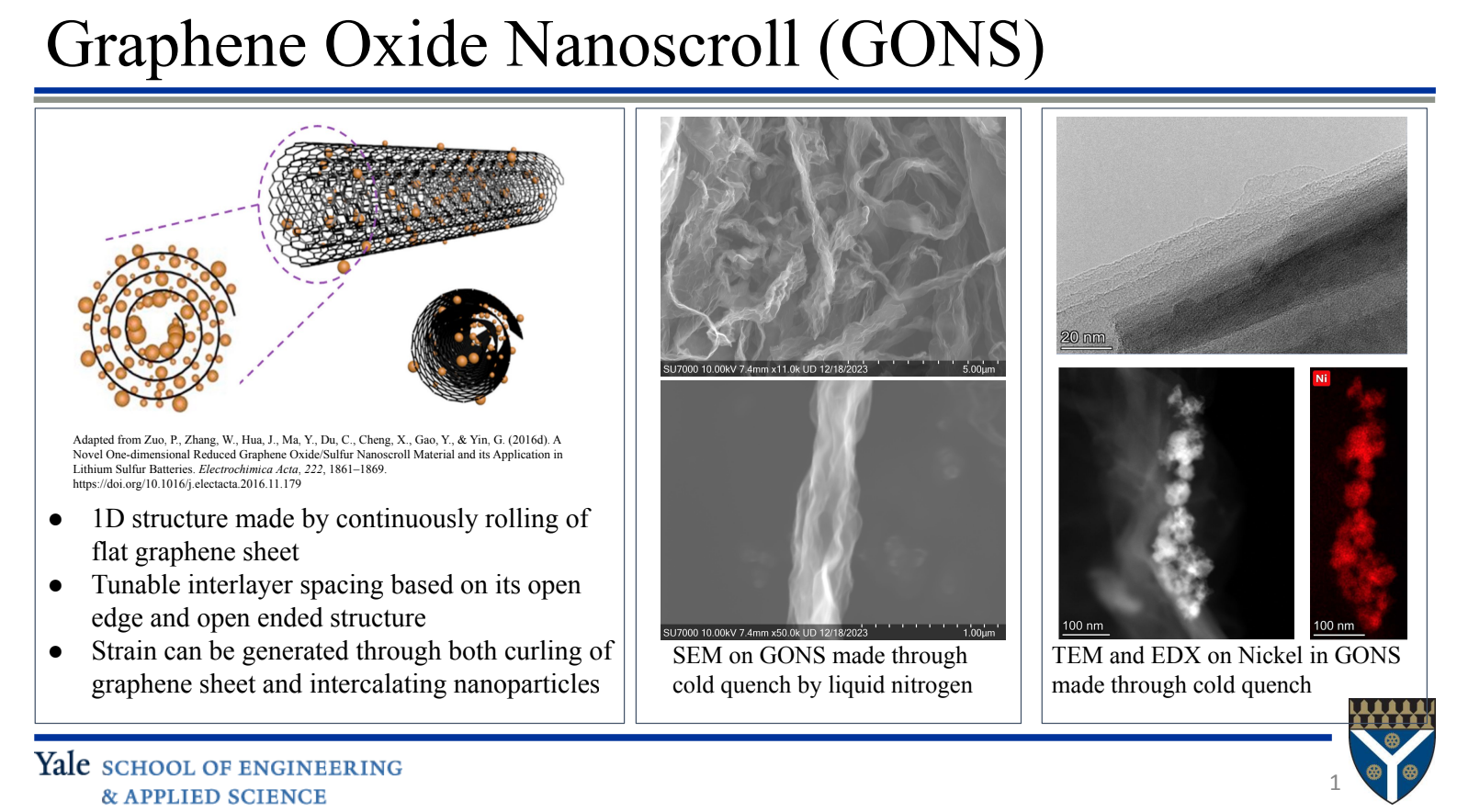
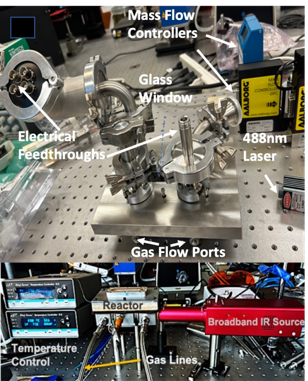
Most important catalytic reactions in nature occur under nano-confined conditions. In living organisms, compartmentalisation within cells and organelles drives catalysis of naturally occurring reactions. All well-known industrial heterogeneous catalytic systems pale in comparison with those in nature with regard to their efficiency and selectivity. Hence, fundamental understanding of how catalysts are affected by a confining environment could provide some remarkable new insights and scientific discoveries [‘Confined catalysis under two-dimensional materials’ by Li, Xiao, Qiang and Bao, PNAS, Vol 114, 5930-5934, June 6, 2017]. The interactions brought about by confinement are complex and their coupling makes interpretation and first principles study difficult. In our current work (funded by the Army Research Office) we intend to apply new experimental techniques for in operando analysis of the state of the catalyst, adsorbates and products.
We are currently looking at linked graphene oxide based systems where the catalyst is intercalated between the sheets or scrolls of the 2D materials for direct conversion of CO 2 to ethane and/or ethylene. Future work involves using hexagonal Boron Nitride as a confining vessel and look at other reactions which are important for mitigating the impact of climate change.
A parallel effort is the study of photothermal reactions using nanoparticles like silver for catalytic conversion. Hematite (α-Fe2O3), the most thermodynamically stable form of iron oxides, is a highly effective catalyst for the complete oxidation of methane with excellent stable performance below 500 °C in both nano and bulk forms, a low light-off temperature of 230 °C, and 100% selectivity to CO2. Our research has demonstrated that modification of hematite with silver nanoparticles has promising applications for conversion of methane to useful products such as methanol.